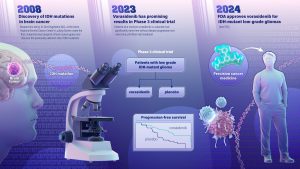
The FDA has approved a new drug treatment for a type of brain cancer called low-grade glioma. The drug, called vorasidenib, is a targeted cancer therapy that works by inhibiting the activity of a mutated gene called IDH, slowing the growth of the cancer. IDH was first identified by Dr Bert Vogelstein and his team at the Johns Hopkins Kimmel Cancer Center’s Ludwig Center. Read more about the FDA approval here.
Watch Dr Vogelstein explain the significance of the approval of this drug treatment.